MW Aims for Sustainable Medical Care Regime
Strives to ramp up Proactive health management services
by establishing a system to prevent and manage super bacteria and epidemics

New Drugs Developed in Korea
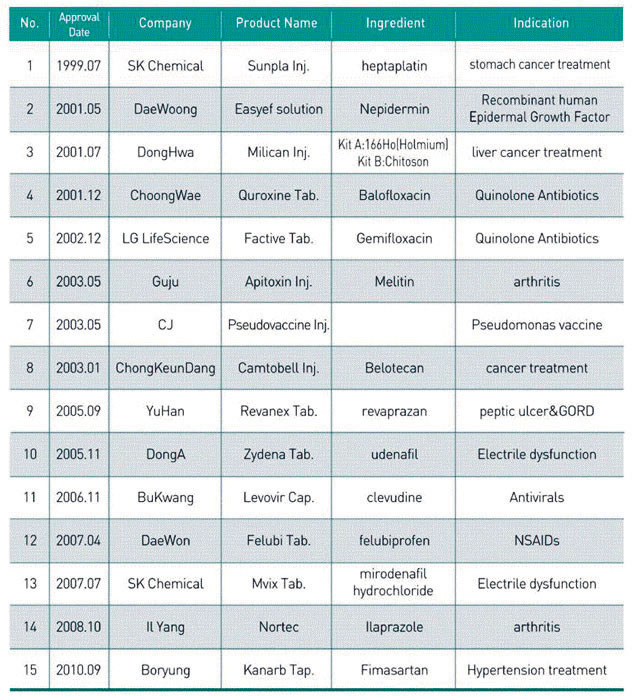
Source: the Ministry of Health and Welfare
The government plans to establish a blueprint for the future development of healthcare systems, calling for the building of a sustainable healthcare regime, Sohn Gunn-Yik, assistant minister of the Office for Healthcare Policy at the Ministry of Health and Welfare (MW), said.
The following are the excerpts of an interview between NewsWorld and MW Deputy Minister Sohn in which he spoke about his office's policies on the provision of medical treatment, the nurturing of the medical industry and the development of new medicines.
Question: Will you tell our readers about major tasks your office will implement this year?
Answer: First of all, our office carries out detailed tasks designed to build a sustainable healthcare regime, and we plan to present by August a blueprint for the future development of healthcare systems.
The Office for Healthcare Policy strives to provide quality medical services by enhancing the efficient management of medical resources ¡ª hospital beds, manpower and medical equipment ¡ª and establishing a chronic disease management system through the reinvigorating of neighborhood clinics.
We're ramping up a medical safety net for low-income people by expanding the beneficiaries of new health insurance benefit coverage to 1.35 million people at a cost of 331.9 billion won in 2011, establishing a Korean medical support foundation for the underprivileged, supporting the establishment of obstetrics clinics in vulnerable areas for childbirth and introducing medical helicopters for emergency patients.
The government is striving to expand or ramp up the provision of proactive health management services by establishing an advanced system to prevent and manage super bacteria and new epidemics and by strengthening controls on habitual smoking, alcoholism and depression and other causes of health hazards.
In an effort to help the domestic health and medical industry, emerging as a new growth engine in the Post-IT era, and make inroads into the global market, our office is executing such strategies as the invigorating of attracting foreign patients, joint exporting by hospitals, the IT sector, medical practitioners and the medical equipment industry, the operating of research-oriented hospitals, establishment of an advanced comprehensive medical industrial complex and the implementation of a strategy to tap the North American market, dubbed the Columbus Project.
Q: What steps is your ministry taking regarding the ongoing controversy of making such pharmaceuticals as cold medicines available over the counter in supermarkets and other places?
A: Our ministry is seeking to revise the Act on Pharmacists so as to ease the inconveniences people experience when trying to buy medicine in the middle of the night or on holidays.
To this end, an experts' review into determining whether some prescription medicines will be available for purchase at places other than pharmacies, an expert seminar and a public hearing designed to gather public opinions have been conducted recently.
The ministry has put on public review a bill on the projected amendment on July 28. It plans to submit the measure to the National Assembly for approval around the end of September.
Initially, the government is doing its utmost to have the National Assembly act on the revision bill, a measure to alleviate the public inconveniences they experience when they purchase medicines, and gain approval on the bill from the parliament by holding consultation meetings with the ruling party and by persuading lawmakers.
Q: Will you elaborate on offering incentives to institutions that purchase medicines at cheaper prices and penalizing both sides who give and receive rebates?
A: A market-oriented real price system to eradicate rebates related to the marketing of medical products and give incentives to those who purchase them at cheaper prices took effect last October.
We continue to monitor the system so that it can work. The ministry took another supplementary step to provide financial support to ensure a stable supply of essential medicines this past February.
A system of punishing both those in the medical field and pharmacists found to have engaged in illegal practices related to the marketing of pharmaceuticals and medical equipment was put into practice starting last Nov. 28.
In an effort to probe into some illegal rebate dealings involving a pending change of patented drugs into generic medicines, a government joint investigation team on giving and receiving rebates on medical products was inaugurated at the Seoul District Prosecutors' Office. Our ministry is jointly cooperating with the Fair Trade Commission and the National Tax Service to form a regular surveillance regime to ensure the fair trade of pharmaceuticals.
Q: Will you tell us about the current status of new medicines Korean pharmaceutical companies are developing?
A: There are 15 new drugs that were developed in Korea and put on the market, all of which have been approved by the Korea Food Drug Administration (KFDA). They include Sunpla, developed by SK Chemical and approved by the KFDA in July 1999.
Q: Will you be more specific on plans to obtain vaccines for H5 influenzas?
A: We're striving to develop diagnosis and surveillance technology as well as vaccines for H5 influenza in humans that breaks out constantly in Southeast Asia and Africa.
The Korea Centers for Disease Control and Prevention (KCDC), under the umbrella of the MW, is conducting the second clinical test of vaccines for H5 influenza in humans that are now under development.
An inter-ministry new influenza business team, inaugurated in 2010, is now working on the development of vaccines for H5 influenza through the commercialization of advanced cell culture vaccine development technologies.
Q: Will you explain the policies to upgrade medical institutions to the standards of advanced countries and allow for-profit hospitals?
A: The government has a unified stance stressing the need for permitting for-profit hospitals in which foreign investors will be allowed to take a more than 50 percent stake with the goal of building a favorable investment and residence environment enticing foreign investors in free economic zones and Jeju Special Self-governing Province. But the reality is that public consensus has not been reached on the full-fledged introduction of for-profit medical institutions.
In this regard, the government needs to take up the full-fledged introduction of for-profit medical institutions in consideration of the expansion of public medical care levels and health insurance coverage levels in the mid- and long-term perspective after taking stock of the operations of the for-profit hospitals being inaugurated in the FEZ and Jeju areas under the related acts.
Q: Will you specify the current status of attracting foreign patients here and strategies to boost foreign visitors who receive medical treatment in Korea?
A: A program to lure foreign patients that began in 2009 is now in an early state. But the number of foreign visitors on medical tour here surged 36 percent to 81,789 in 2010 from 60,201 in 2009 while tourism trade deficit for medical treatment reduced from $69.8 million in 2007 to $59.2 million in 2008 and $13.2 million in 2009 before turning to a $2.2 million surplus in 2010.
The domestic medical treatment field has a long way to go, however. The nation has insufficient infrastructure for attracting foreign patients to Korea such as low global standing of Korea's medical service and language barriers despite lower medical costs and excellent medical service technology.
In a bid to stimulate the attraction of foreign patients, the government plans to establish and implement the Strategy for Advancement : Phase II to transform Korea into a hub of medical tourism in Asia with the goal of attracting 110,000 foreign patients in 2011 and 300,000 inbound tourists on medical tour in 2015. The plan calls for putting into practice top seven priority tasks the introducing of a compensation system for foreign patients; the easing of regulations on the construction of accommodation facilities within medical institutions in the Seoul metropolitan area; allowing hospital pharmacies to prepare prescriptions for foreign patients; widening foreign doctors' opportunities to undergo training and clinical testing programs in Korea; fostering medical service interpreters and other specialized manpower; evaluating infrastructure for attracting foreign patients and simplifying of visa-related paperwork.
Q: Will you speak about policies to ensure food and health supplement food safety?
A: Ensuring the safety of foods is a value of the highest priority that must never be neglected even for a moment.
New demands have continued to develop such as the prevention of food imports tainted with radioactive materials in the wake of the Japanese nuclear accident, the promotion of sound distribution and sales of health supplement foods, the improving of effectiveness of a ban on foods harmful to children in the green food zones and indicating of harmfulness degrees and the need for managing risk factors of obesity in the long-term perspective.
The ministry strives to continuously develop optimal policies to advance the safety of foods to the standards of advanced countries now that it is urgently required to predict and effectively address these demands for protecting and improving public health. nw
Sohn Gunn-Yik, assistant minister of the Office for Healthcare Police at the Ministry of Health and Welfare.
Photo by Newsworld
3Fl, 292-47, Shindang 6-dong, Chung-gu, Seoul, Korea 100-456
Tel : 82-2-2235-6114 / Fax : 82-2-2235-0799